Monday 31 March 2008
Sunday 30 March 2008
Saturday 29 March 2008
Puberty chosen Intent chosen Direction chosen Diabetes ...HOW ?
"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
IS 'SEX' WITHOUT-INTENT-TO-PROCREATE SUSTAINING' DIABETIC' TYPE HYPERGLYCEMIA BY CHRONIC LOSS OF ZINC-CONTAINING SEXUAL FLUIDS?
'CELIBATING' for natural PROTECTION & more stable BLOOD GLUCOSE?
> 0777 www.tinyurl.com/yu3xx9 {Ageing in Drosophilia is modified according to their physiological state. Adult longevity is decreased by sexual activity: in both sexes the life span of virgins is higher. For the same physiological state male longevity is always lower than that of females.}
> 0896 www.tinyurl.com/3b5hsr {Gisela.Dahlquist@pediatri.umu.se ~ Zinc deficiency has shown to increase the risk for diabetes in diabetes-prone experimental animals.}
> 0208 www.tinyurl.com/2dmqk8 {Ian.MrTao@Gmail.com ~ naked PROTECTION}
> 0407 www.tinyurl.com/2odhdo {Masturbation video illustrating extreme sexual fluid loss and concomitant potential ZINC deficit induced alpha-cell HYPERglycemia -- There is a different outcome between alpha-cell glucagon ... stimulated relative-HYPERglycemia [EG a horror movie (www.tinyurl.com/2opc5h)] ~ VS ~ eating too OFTEN ... caused acute relative-HYPERglycemia [EG a cake (www.tinyurl.com/32dvnb) and a combination of both in the form of LOSS of sexual fluids as per the referenced video about masturbation.}
> 0407 www.tinyurl.com/2oonjv {'...We propose that during HYPOglycemia the principal signal that initiates glucagon secretion is the detection by alpha-cells of a sudden decrease in ZINC ... stimulates glucagon secretion.'}
> 0108 www.tinyurl.com/yrd6u {Alpha-cells & Epsilon-cells (www.tinyurl.com/2psoy3) secrete ghrelin which inhibits beta-cell insulin release & stimulates growth hormone release from the anterior pituitary (www.tinyurl.com/3exose) AND alpha-cells secrete glucagon which stimulates glucose production in / release from ... the LIVER ... into the blood.}
> 1007 www.tinyurl.com/2jknrw {Ian Clark ~ CELIBATING ~ Brotherhood & Sisterhood}
> 0108 www.tinyurl.com/38c2ek {"...For the record, I am a practcing Catholic who waited until sex for marriage...unlike Ms. Berry. And yet she is "cured" and not me...go figure..."}
> 0208 www.tinyurl.com/24sls8 {continent ~ VS ~ incontinent ... Masturbation causes BOTH females & males much loss of sexual fluids ... but this article highlights the female potential to actively absorb the male fluids which may hint at potential reason for female / male differences in lifespan.
> 0807 www.tinyurl.com/238xfv {Ian Clark ~ CELIBATING ~ Time}
> 0907 www.tinyurl.com/25qqvy {Ian.MrTao@Gmail.com ~ CELIBATING ~ Contraception / Zinc barrier / HPV}
HOW INCONTINENT SHOULD YOU BE?
Friday 28 March 2008
Body Earth GIA Glucose Insulin Amylin ...HOW shocking ?
"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
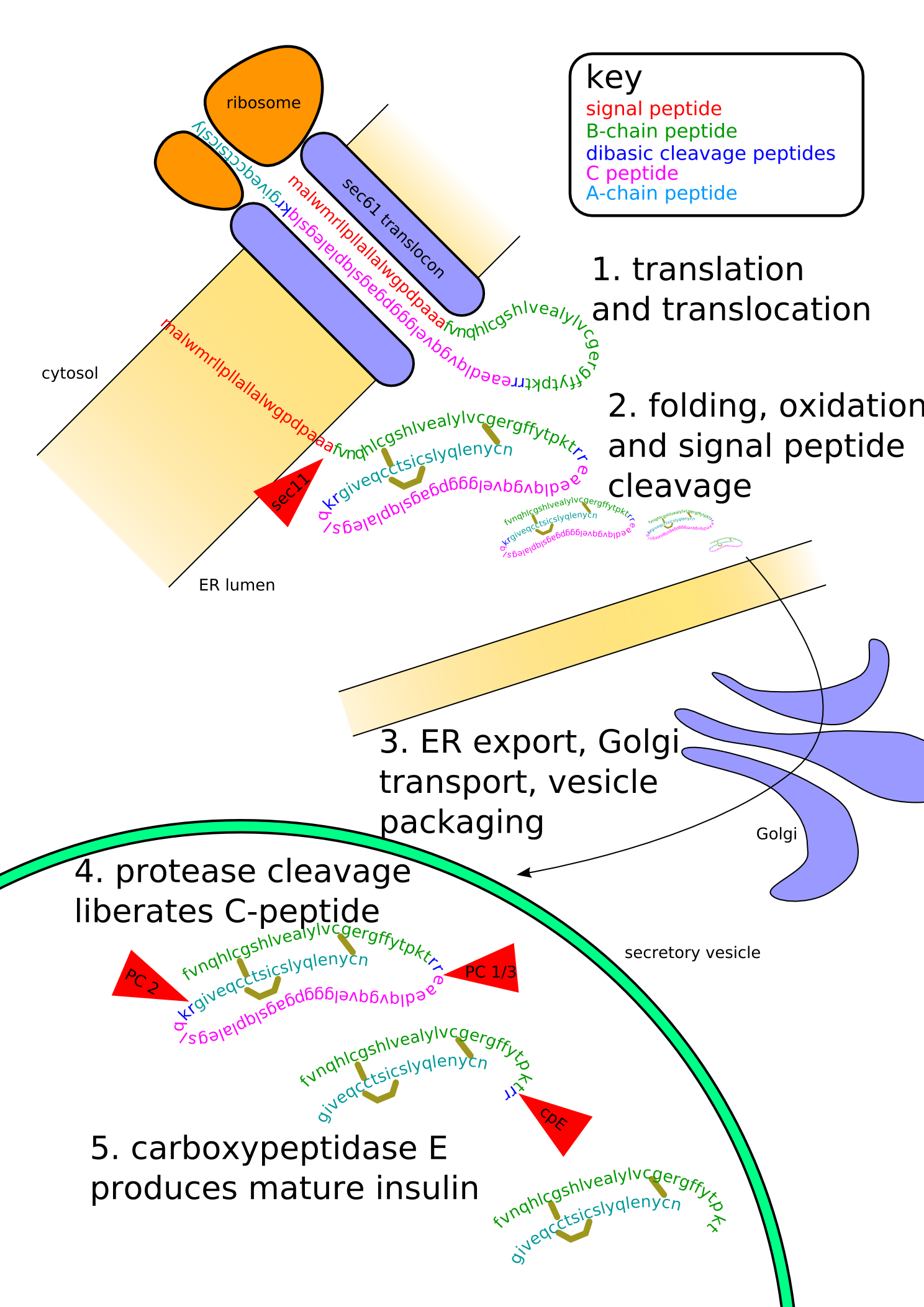
Proinsulin is the prohormone precursor to insulin made in the beta cell of the islets of Langerhans. It is synthesized in the endoplasmic reticulum, where it is folded and its disulfide bonds are oxidized. It is then transported to the Golgi apparatus where it is packaged into secretory vesicles, and where it is processed by a series of proteases to form mature insulin. Mature insulin has 39 fewer amino acids; 4 are removed altogether, and the remaining 35 form the C-peptide. The C-peptide is abstracted from the center of the proinsulin sequence; the two other ends (the B chain and A chain) remain connected by disulfide bonds.
When insulin was originally purified from bovine or porcine pancreata, all the proinsulin was not fully removed. When some people used these insulins, the proinsulin may have caused the body to react with a rash, to resist the insulin, or even to make dents or lumps in the skin at the place where the insulin was injected. In most cases however, the iatrogenic immune response comes from slight differences between species of insulin rather than from proinsulin itself. Since the late 1970's, when highly-purified pork insulin was introduced, and the level of insulin purity reached 99%, this ceased to be a significant clinical issue.
It should also be noted that in respect of their influence on insulin pharmacokinetics, moderate concentrations of certain insulin antibodies may, in fact, be of positive advantage to all diabetics without endogenous insulin secretion (e.g. people with type 1 diabetes) because insulin binding antibodies effectively increase the insulin's clearance rate and distribution space and therefore helps to prolong its pharmacological and biological half lives.
Insulin undergoes extensive posttranslational modification along the production pathway. Production and secretion are largely independent; prepared insulin is stored awaiting secretion. Both C-peptide and mature insulin are biologically active. Cell components and proteins in this image are not to scale...
2006 (C) Isaac Yonemoto
Proamylin Proislet Amyloid Polypeptide (proIAPP,Proamylin, Amyloid Polypeptide Precursor, Proislet Protein) Proislet amyloid polypeptide (proIAPP) is the protein precursor for Islet amyloid polypeptide (IAPP, amylin) (Higham et al., 2001).
ProIAPP is produced in the pancreatic beta cells (β-cells) as a 67 amino acid, 7404 Dalton pro-peptide and undergoes post-translational modifications to become the 37 amino acid IAPP hormone, also known as amylin. IAPP is cosecreted with insulin from the pancreatic β-cells in a ratio of approximately 100:1. Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
Once produced by the β-cells, it undergoes proteolysis. After this the transformation from the precursor protein proIAPP to the biologically active IAPP is complete.
Insulin and IAPP are regulated by similar factors since they share a common regulatory promoter sequence (Höppener, Ahrén, & Cornelius, 2000). One of the defining features of Type 2 diabetes is insulin resistance. This is a condition wherein the body is unable to utilize insulin effectively, resulting in increased insulin production; since proinsulin and proIAPP are cosecreted, this results in an increase in the production of proIAPP as well.
Although little is known about IAPP regulation, its connection to insulin indicates that regulatory mechanism that affect insulin also affect IAPP. Thus blood glucose levels play an important role in regulation of proIAPP synthesis.
ProIAPP has been linked to Type 2 diabetes and the loss of islet β-cells (Paulsson et al., 2005). Islet amyloid formation, initiated by the aggregation of proIAPP, may contribute to this progressive loss of islet β-cells. It is thought that proIAPP forms the first granules that allow for IAPP to aggregate and form amyloid which may lead to amyloid-induced apoptosis of β-cells.
IAPP is cosecreted with insulin. Insulin resistance in Type 2 diabetes produces a greater demand for insulin production which results in the secretion of proinsulin (Marzban, Rhodes, Haataja, Halban, & Verchere, 2006). ProIAPP is secreted simultaneously, however, the enzymes that convert these precursor molecules into insulin and IAPP, respectively, are not able to keep up with the high levels of secretion, ultimately leading to the accumulation of proIAPP.
In particular, the impaired processing of proIAPP that occurs at the N-terminal cleavage site is a key factor in the initiation of amyloid (Marzban et al., 2006). Post-translational modification of proIAPP occurs at both the carboxy terminus and the amino terminus, however, the processing of the amino terminus occurs later in the secretory pathway. This might be one reason why it is more susceptible to impaired processing under conditions where secretion is in high demand (Marzban et al., 2005). Thus, the conditions of Type 2 diabetes—high glucose concentrations and increased secretory demand for insulin and IAPP—could lead to the impaired N-terminal processing of proIAPP. The unprocessed proIAPP can then serve as the granule upon which IAPP can accumulate and form amyloid (Paulsson et al., 2006).
The amyloid formation might be a major mediator of apoptosis, or programmed cell death, in the islet β-cells (Paulsson et al., 2006). Initially, the proIAPP aggregates inside the cell. The proIAPP acts as a seed, collecting IAPP from neighboring cells and forming an intracellular amyloid. As the amyloid grows, it spreads outside of the cell. The extracellular amyloid begins to excrete IAPP to other cells, inducing similar amyloid formation in other β-cells. The overall effect is an apoptosis cascade initiated by the influx of ions into the β-cells.
In summary, impaired N-terminal processing of proIAPP is an important factor initiating amyloid formation and β-cell death. These amyloid deposits are pathological characteristics of the pancreas in Type 2 diabetes. However, it is still unclear as to whether amyloid formation is involved in or merely a consequence of type 2 diabetes (Marzban et al., 2006). Nevertheless it is clear that amyloid formation reduces working β-cells in patients with Type 2 diabetes. This suggests that repairing proIAPP processing may help to prevent β-cell death, thereby offering hope as a potential therapeutic approach for Type 2 diabetes.
Amylin, or Islet Amyloid Polypeptide (IAPP), is a 37-residue peptide hormone secreted by pancreatic β-cells at the same time as insulin (in a roughly 100:1 ratio).
Islet, or insulinoma, amyloid polypeptide (IAPP, or amylin) is commonly found in pancreatic islets of patients suffering diabetes mellitus type II, or harboring an insulinoma. While the assosciation of amylin with the development of type II diabetes has been known for some time, a direct causative role for amylin has been harder to establish. Recent results suggest that amylin, like the related beta-amyloid (Abeta) assosciated with Alzheimer's disease, can induce apoptotic cell-death in particular cultured cells, an effect that may be relevant to the development of type II diabetes.[1]
Amylin functions as part of the endocrine pancreas and contributes to glycemic control. Amylin's metabolic function is now somewhat well characterized as an inhibitor of the appearance of nutrient [especially glucose] in the plasma. It thus functions as a synergistic partner to insulin, with which it is cosecreted from pancreatic beta cells in response to meals. The overall effect to slow the rate of appearance (Ra) from the meal is mediated via a coordinate reduction of food intake, slowing of gastric emptying, inhibition of digestive secretion [gastric acid, pancreatic enzymes, and bile ejection].
Appearance of new glucose is slowed by inhibiting secretion of the gluconeogenic hormone glucagon. These actions, which are mostly mediated via a glucose-sensitive part of the brain stem, the area postrema, may be over-ridden during HYPOglycemia.
They collectively reduce the total insulin demand.[2][3]
Rodent amylin knockouts are known to fail to achieve the normal anorexia following food consumption. Because it is an amidated peptide, like many neuropeptides, it is believed to be responsible for the anorectic effect.
The human form of IAPP has the amino acid sequence KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY, with a disulfide bridge between cysteine residues 2 and 7. The peptide is secreted from the pancreas into the blood circulation and eventually excreted by the kidneys.
IAPP is capable of forming amyloid fibrils in vitro. Within the fibrillization reaction, the early prefibrillar structures are extremely toxic to insuloma cells cultures. Later amyloid fibril structures also seem to have some cytotoxic effect on cell cultures. Rats and mice have six substitutions (three of which are proline substitions at positions 25, 28 and 29) that are believed to prevent the formation of amyloid fibrils. Rat IAPP is unique since it is the only non fibril forming IAPP form.
IAPP was identified independently by two groups as the major component of diabetes-associated islet amyloid deposits in 1987.[4][5]
Synthetic amylin, or pramlintide (brand name Symlin), was recently approved for adult use in patients with both diabetes mellitus type 1 and diabetes mellitus type 2. Insulin and pramlintide, injected separately but both before a meal, work together to control the post-prandial glucose excursion.
There appears to be at least three distinct receptor complexes that bind with high affinity to amylin. All three complexes contain the calcitonin receptor at the core, plus one of three Receptor activity-modifying proteins, RAMP1, RAMP2, or RAMP3.
Thursday 27 March 2008
Why LOVE cholesterol and LOVE diabetes ...HOW ?
"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
Proinsulin is the prohormone precursor to insulin made in the beta cell of the islets of Langerhans. It is synthesized in the endoplasmic reticulum, where it is folded and its disulfide bonds are oxidized. It is then transported to the Golgi apparatus where it is packaged into secretory vesicles, and where it is processed by a series of proteases to form mature insulin. Mature insulin has 39 fewer amino acids; 4 are removed altogether, and the remaining 35 form the C-peptide. The C-peptide is abstracted from the center of the proinsulin sequence; the two other ends (the B chain and A chain) remain connected by disulfide bonds.
When insulin was originally purified from bovine or porcine pancreata, all the proinsulin was not fully removed. When some people used these insulins, the proinsulin may have caused the body to react with a rash, to resist the insulin, or even to make dents or lumps in the skin at the place where the insulin was injected. In most cases however, the iatrogenic immune response comes from slight differences between species of insulin rather than from proinsulin itself. Since the late 1970's, when highly-purified pork insulin was introduced, and the level of insulin purity reached 99%, this ceased to be a significant clinical issue.
It should also be noted that in respect of their influence on insulin pharmacokinetics, moderate concentrations of certain insulin antibodies may, in fact, be of positive advantage to all diabetics without endogenous insulin secretion (e.g. people with type 1 diabetes) because insulin binding antibodies effectively increase the insulin's clearance rate and distribution space and therefore helps to prolong its pharmacological and biological half lives.
Insulin undergoes extensive posttranslational modification along the production pathway. Production and secretion are largely independent; prepared insulin is stored awaiting secretion. Both C-peptide and mature insulin are biologically active. Cell components and proteins in this image are not to scale...
2006 (C) Isaac Yonemoto
Proamylin Proislet Amyloid Polypeptide (proIAPP,Proamylin, Amyloid Polypeptide Precursor, Proislet Protein) Proislet amyloid polypeptide (proIAPP) is the protein precursor for Islet amyloid polypeptide (IAPP, amylin) (Higham et al., 2001).
ProIAPP is produced in the pancreatic beta cells (β-cells) as a 67 amino acid, 7404 Dalton pro-peptide and undergoes post-translational modifications to become the 37 amino acid IAPP hormone, also known as amylin. IAPP is cosecreted with insulin from the pancreatic β-cells in a ratio of approximately 100:1. Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
Once produced by the β-cells, it undergoes proteolysis. After this the transformation from the precursor protein proIAPP to the biologically active IAPP is complete.
Insulin and IAPP are regulated by similar factors since they share a common regulatory promoter sequence (Höppener, Ahrén, & Cornelius, 2000). One of the defining features of Type 2 diabetes is insulin resistance. This is a condition wherein the body is unable to utilize insulin effectively, resulting in increased insulin production; since proinsulin and proIAPP are cosecreted, this results in an increase in the production of proIAPP as well.
Although little is known about IAPP regulation, its connection to insulin indicates that regulatory mechanism that affect insulin also affect IAPP. Thus blood glucose levels play an important role in regulation of proIAPP synthesis.
ProIAPP has been linked to Type 2 diabetes and the loss of islet β-cells (Paulsson et al., 2005). Islet amyloid formation, initiated by the aggregation of proIAPP, may contribute to this progressive loss of islet β-cells. It is thought that proIAPP forms the first granules that allow for IAPP to aggregate and form amyloid which may lead to amyloid-induced apoptosis of β-cells.
IAPP is cosecreted with insulin. Insulin resistance in Type 2 diabetes produces a greater demand for insulin production which results in the secretion of proinsulin (Marzban, Rhodes, Haataja, Halban, & Verchere, 2006). ProIAPP is secreted simultaneously, however, the enzymes that convert these precursor molecules into insulin and IAPP, respectively, are not able to keep up with the high levels of secretion, ultimately leading to the accumulation of proIAPP.
In particular, the impaired processing of proIAPP that occurs at the N-terminal cleavage site is a key factor in the initiation of amyloid (Marzban et al., 2006). Post-translational modification of proIAPP occurs at both the carboxy terminus and the amino terminus, however, the processing of the amino terminus occurs later in the secretory pathway. This might be one reason why it is more susceptible to impaired processing under conditions where secretion is in high demand (Marzban et al., 2005). Thus, the conditions of Type 2 diabetes—high glucose concentrations and increased secretory demand for insulin and IAPP—could lead to the impaired N-terminal processing of proIAPP. The unprocessed proIAPP can then serve as the granule upon which IAPP can accumulate and form amyloid (Paulsson et al., 2006).
The amyloid formation might be a major mediator of apoptosis, or programmed cell death, in the islet β-cells (Paulsson et al., 2006). Initially, the proIAPP aggregates inside the cell. The proIAPP acts as a seed, collecting IAPP from neighboring cells and forming an intracellular amyloid. As the amyloid grows, it spreads outside of the cell. The extracellular amyloid begins to excrete IAPP to other cells, inducing similar amyloid formation in other β-cells. The overall effect is an apoptosis cascade initiated by the influx of ions into the β-cells.
In summary, impaired N-terminal processing of proIAPP is an important factor initiating amyloid formation and β-cell death. These amyloid deposits are pathological characteristics of the pancreas in Type 2 diabetes. However, it is still unclear as to whether amyloid formation is involved in or merely a consequence of type 2 diabetes (Marzban et al., 2006). Nevertheless it is clear that amyloid formation reduces working β-cells in patients with Type 2 diabetes. This suggests that repairing proIAPP processing may help to prevent β-cell death, thereby offering hope as a potential therapeutic approach for Type 2 diabetes.
Amylin, or Islet Amyloid Polypeptide (IAPP), is a 37-residue peptide hormone secreted by pancreatic β-cells at the same time as insulin (in a roughly 100:1 ratio).
Islet, or insulinoma, amyloid polypeptide (IAPP, or amylin) is commonly found in pancreatic islets of patients suffering diabetes mellitus type II, or harboring an insulinoma. While the assosciation of amylin with the development of type II diabetes has been known for some time, a direct causative role for amylin has been harder to establish. Recent results suggest that amylin, like the related beta-amyloid (Abeta) assosciated with Alzheimer's disease, can induce apoptotic cell-death in particular cultured cells, an effect that may be relevant to the development of type II diabetes.[1]
Amylin functions as part of the endocrine pancreas and contributes to glycemic control. Amylin's metabolic function is now somewhat well characterized as an inhibitor of the appearance of nutrient [especially glucose] in the plasma. It thus functions as a synergistic partner to insulin, with which it is cosecreted from pancreatic beta cells in response to meals. The overall effect to slow the rate of appearance (Ra) from the meal is mediated via a coordinate reduction of food intake, slowing of gastric emptying, inhibition of digestive secretion [gastric acid, pancreatic enzymes, and bile ejection].
Appearance of new glucose is slowed by inhibiting secretion of the gluconeogenic hormone glucagon. These actions, which are mostly mediated via a glucose-sensitive part of the brain stem, the area postrema, may be over-ridden during HYPOglycemia.
They collectively reduce the total insulin demand.[2][3]
Rodent amylin knockouts are known to fail to achieve the normal anorexia following food consumption. Because it is an amidated peptide, like many neuropeptides, it is believed to be responsible for the anorectic effect.
The human form of IAPP has the amino acid sequence KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY, with a disulfide bridge between cysteine residues 2 and 7. The peptide is secreted from the pancreas into the blood circulation and eventually excreted by the kidneys.
IAPP is capable of forming amyloid fibrils in vitro. Within the fibrillization reaction, the early prefibrillar structures are extremely toxic to insuloma cells cultures. Later amyloid fibril structures also seem to have some cytotoxic effect on cell cultures. Rats and mice have six substitutions (three of which are proline substitions at positions 25, 28 and 29) that are believed to prevent the formation of amyloid fibrils. Rat IAPP is unique since it is the only non fibril forming IAPP form.
IAPP was identified independently by two groups as the major component of diabetes-associated islet amyloid deposits in 1987.[4][5]
Synthetic amylin, or pramlintide (brand name Symlin), was recently approved for adult use in patients with both diabetes mellitus type 1 and diabetes mellitus type 2. Insulin and pramlintide, injected separately but both before a meal, work together to control the post-prandial glucose excursion.
There appears to be at least three distinct receptor complexes that bind with high affinity to amylin. All three complexes contain the calcitonin receptor at the core, plus one of three Receptor activity-modifying proteins, RAMP1, RAMP2, or RAMP3.
Wednesday 26 March 2008
fda HYPO-intolerant reaction choice now or HYPER-intolerant LIFEstyle - You choose your LIVER reaction and see what LOVE-diabetes really is ...HOW ?
"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
Eating less OFTEN is profoundly more healthy than eating less...
> 0400 www.tinyurl.cOM/2j7p3t [DFadool@neuro.fsu.edu]
> 0501 www.tinyurl.cOM/yqf8gj [Maeda@ys7.u-shizuoka-ken.ac.jp]
> 0503 www.tinyurl.cOM/ys63gk [Anson@jhu.edu]
> 0407 www.tinyurl.cOM/29kvda [ZangenD@hadassah.org.il]
> 1007 www.tinyurl.cOM/3aypqg [MRistow@mristow.org]
.
Glucose / insulin "resistance" arises in at least 2 forms: (A) Insulin receptors which adaptively reduce their functional efficiency of 'pumping' glucose into cells; and (B) Insulin receptors that 'disappear' from cells. Why do You think there is just 1 name, in common use, to describe more than 2, vitally different, physiologies?? Type 1 & type 2 Diabetic ... glucose / insulin 'resistance' arises primarily via insulin receptors which adaptively down-regulate their rate of 'pumping' glucose into fat / liver / muscle cells ... so that more glucose is diverted/available for the brain / nervous system.
http://tinyurl.com/3aypqg [mristow@mristow.org]
Type 3 Diabetic ... glucose / insulin 'resistance' arises primarily via insulin receptors that 'disappear', from nerve cells, BECAUSE eating too OFTEN results in 'cycles' of localised brain / nerve damage & 'sclerosis scarring' [eg called 'Alzheimers' & 'peripheral neuropathy' ] via "relative-HYPOglycemia" ... which subsequently results in localised brain/nerve starvation c/o the 'glucose-starved HYPOglycemia', primary stage, followed by localised brain/nerve destruction during the "relative-HYPERglycemia", secondary stage, also called 'glucose re-perfusion'.
http://tinyurl.com/ynpp4g [raymond.swanson@ucsf.edu]
... In the absence of adaptative [protective] type 3 Diabetic glucose / insulin 'resistance' [aka brain-insulin receptor down-regulation] ... too OFTEN meal-induced 'glucose re-perfusion', via highly efficient nerve-insulin receptors, could induce massive neural damage relatively quickly [cf. youth-onset neuropathy].
http://tinyurl.com/yuh3q8 [mattisonj@mail.nih.gov]
Type 3 diabetes Is Caused By Food And Or Drug Administration Too Much And Or Too Often.
http://tinyurl.com/yta44e [giulio.passinetti@mssm.edu]
Pancreatic-insulin or 'digestion-insulin' [primarily for STORING glucose in fat, liver & muscle cells] for FUTURE use by the brain & nervous system] is usually produced by beta-cells following a period of eating. Brain-insulin or 'nerve-insulin' [primarily for CURRENT use to help with REGULATING glucose feeding into the nervous system] is produced by 'nerve cells' following a suitable period of sleep/eating less OFTEN.
http://www.tinyurl.com/2j7p3t [dfadool@neuro.fsu.edu]
Eating less OFTEN increases the number of brain-insulin receptors, increases the availability of glucose to the brain, reduces appetite and increases the availability of ketone fuel for the brain.
http://tinyurl.com/ys63gk [anson@jhu.edu]
Eating too often decreases the number of brain-insulin receptors, reduces the availability of glucose to the brain, increases appetite and reduces the availability of ketone fuel for the brain.
http://tinyurl.com/26jy4l [jens.bruening@uni-koeln.de]
Eating less is profoundly different from eating less OFTEN.
http://tinyurl.com/323nj3 [froelich@em.uni-frankfurt.de]
Blood glucose feeds the brain efficiently in the absence of any pancreatic-insulin because in the absence of food [ie following a sufficient period of sleep/fasting] the brain-insulin production is increased to help 'pump' the relatively lower levels of blood glucose into the nerves eg into the brain via brain-insulin receptors.
http://www.tinyurl.com/yr48jq [freychet@unice.fr]
Type 1, 2 or 3 Diabetes is NOT a disease [it is the associated 'HYPOglycemia' that is the 'disease'] ... type 1 & type 2 diabetes aka HYPERglycemia [that can exist for years] is a 'physiological body state' that protects the nervous system [especially the brain] from the extraordinary dangers of beta-cell and/or HYPERinsulinemia [excess insulin] associated "relative-HYPOglycemia" [which starves the nervous system of fuel and can result in profound challenges within minutes, or even seconds, with little or zero notice].
http://tinyurl.com/yu77y7 [m.henricsson@telia.com]
Type 1, 2 or 3 diabetes is NOT a disease … Type 1, 2 or 3 diabetes is the CURE [for relative-HYPOglycemia].
http://www.tinyurl.com/yqf8gj [maeda@ys7.u-shizuoka-ken.ac.jp]
'Relative-HYPOglycemia As A Cause Of Neuropsychiatric Illness' @ Journal Of The National Medical Association @ Harry M Salzer MD @ January 1966 @ Vol 58 @ Number 1 @ Table 1 @ Figure 2.In my opinion UNdrugTREATED type 2 diabetes [aka "cellular dietary restriction"] is a protective adaptative response which, by means of protecting the brain from "relative-HYPOglycemia", can be expected to reduce or eliminate type 3 diabetes aka 'Alzheimers'.
http://tinyurl.com/2farm9 [temorgan@usc.edu]
Please ask your current Specialist for advice including the provision of supporting Peer reviewed references evidencing their understanding of this important matter.
http://tinyurl.com/2lwbro [DrBernarr@aol.com]
…Warm thanks; Nick Gracey, BSc(Hons) Medical Biochemistry, Birmingham University, UK, WATerian c/o http://www.diabeteshealth.com/ @ 15:33hrs MON.03.DEC.2007.
www.DiabetesHealth.com/read/2007/12/03/5558.html
http://HealSelf.org/Diabetes.html [DrBernarr@aol.com]
http://tinyurl.com/3cpy57 [wei-qin_zhao@northwestern.edu]
http://tinyurl.com/98bf2 [Suzanne_DeLaMonte_MD@Brown.edu]
http://tinyurl.com/3aypqg [mristow@mristow.org]
http://tinyurl.com/ynpp4g [raymond.swanson@ucsf.edu]
http://tinyurl.com/yuh3q8 [mattisonj@mail.nih.gov]
http://tinyurl.com/yta44e [giulio.passinetti@mssm.edu]
http://tinyurl.com/2j7p3t [dfadool@neuro.fsu.edu]
http://tinyurl.com/ys63gk [anson@jhu.edu]
http://tinyurl.com/26jy4l [jens.bruening@uni-koeln.de]
http://tinyurl.com/323nj3 [froelich@em.uni-frankfurt.de]
http://tinyurl.com/yr48jq [freychet@unice.fr]
http://tinyurl.com/yqf8gj [maeda@ys7.u-shizuoka-ken.ac.jp]
http://tinyurl.com/yu77y7 [m.henricsson@telia.com]
http://tinyurl.com/2farm9 [temorgan@usc.edu]
http://tinyurl.com/2lwbro [DrBernarr@aol.com]
.
Tuesday 25 March 2008
Monday 24 March 2008
Sunday 23 March 2008
Saturday 22 March 2008
Friday 21 March 2008
Why SOy should be fermented and organic ONLY eg Miso Natto Shoyu Tempeh ... and thyroid - diabetes reactions ... HOW ?
"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
Review by Mo Rapier, WATerian ...
I used to put a scoop of SOy powder in orange juice before walking every morning. I felt dizzy and my pulse rate dropped. Once I forced myself to eat a SOy burger because I had heard that SOy was good for you. I got very weak and dizzy. When I stood up, my legs buckled under me like rubber. I stopped eating SOy after that...
Dr. Joseph Mercola (www.Mercola.cOM) says - 'there is a thyroid problem / SOy associated epidemic' ... Most SOy is GMo (genetically modified) unless it says “organic”. Non fermented SOy should be avoided (including Tofu). Fermented SOy (Tempeh, Miso, and Natto) is probably okay. SOy contains Trypsin inhibitors which interfere with protein digestion. SOy is loaded with goitergens which impair thyroid function. SOy contains phytic acid which impairs the ability to absorb minerals. SOy contains phytoestrogens. SOy infant formulas should be avoided. They have found 20,000 times the amount of estrogen in the blood of babies on SOy infant formula compared to babies not on SOy. SOy formula contains toxic levels of manganese and aluminum. SOy protein isolate (aka textured vegetable protein) has high levels of MSG and aluminum.
Deborah Koons Garcia (Massachusetts School of Law)--believes that the increase in allergies and asthma in children may be caused by SOy and other GMo foods.
Dr. Sherrill Sellman, MD states -- Asians consume very little SOy. SOy was never considered a nutritional food. It was only consumed in times of famine when there was nothing else to eat. Asians eat fermented SOy which is easier to digest. The Chinese eat only 2 teaspoons of fermented SOy a day. The Japanese eat 2 tablespoons a day. SOy is dangerous—It is one of the top most allergenic foods. SOy is cross reactive to wheat. SOy causes a range of inflammatory conditions. It is goitergenic. One glass of SOy milk (4 tbsp of SOy) affects the thyroid causing hypothyroidism. SOy increases the need for vitamin D. It is an inefficient form of B12. It has a trypsin inhibitor which blocks the absorption of protein. SOy interferes with the function of the pancreas. SOy is estrogenic. Babies on SOy formula are taking the equivalent of 5 birth control pills a day. The estrogens in SOy cause early puberty in children and reproductive abnormalities. SOy is not recognized by the government as GRAS (Generally recognized as safe). The request for GRAS labeling was denied.
Dr. Alan Greene MD says—SOy ingredients are in a vast array of our foods. 2/3 of fat consumed comes from SOybean oil. SOy protein islolate comes from SOy meal. 98% is fed to livestock. SOy is contaminated with organophophate pesticides. 87% of SOy is GMo. Only use certified organic SOy.
SO (why?) is something SO high in potential for allergic reactions as SOy consumed at all?
The following is an excerpt from--
THERE IS A CURE FOR DIABETES by Gabriel Cousens MD
SOy is also a diabetogenic [diabetes causing] and kaphagenic [mucous causing] food. It is a low-mineral food that robs the body of minerals. About 90 percent of all SOy is genetically modified (GMO). SOy is also one of the top seven allergens, and is widely known to cause immediate hypersensitivity reactions. While in the last forty years SOy has occupied an important place in the transition from an unhealthy meat-based diet to vegetarian and vegan cuisine, it is time for us to upgrade our food choice to one having more benefits, and fewer negative possibilities. In 1986, Stuart Berger MD, placed SOy among the seven top allergens—one of the “sinister seven.
Berger, S. Dr. Berger’s Immune Power Diet. New York: New American Library, 1986.
>0308 The process of fermenting SOy dramatically reduces its potential for causing allergic reactions by as much as 99 percent. During the fermentation process, large proteins are broken down into smaller pieces. These smaller pieces are not recognized by the antibodies that produce the allergic reaction.
http://www.sciencedaily.com/releases/2008/03/080306113750.htm
http://articles.mercola.com/sites/articles/archive/2008/03/27/why-this-type-of-soy-is-better.aspx
>0308 ‘SOy milk formulas comply with a vegetarian diet, but vegetarian diets are generally not recommended for infants… There is no good evidence that SOy helps in colic, loose stools, stomach upset, vomiting, or any belly discomfort… If a baby is allergic to cow milk proteins they are often allergic to SOy milk proteins also… Hydrosylate formulas are a better choice for milk protein allergies in most cases.’
Dr. Michael Davis MD http://www.youtube.com/watch?v=Bg5DmYQQ
>0108 Eliminating SOy from the diet can be difficult for vegans eating processed foods. Many pre-packaged and prepared foods contain hidden SOy listed as “textured vegetable protein” (TVP), “textured plant protein,” “hydrolyzed vegetable protein” (HVP), “vegetable oil,” or “MSG” (monosodium glutamate), “lecithin,” “vegetable broth,” “bouillon,” “natural flavor,” or “mono-diglycerides.” Vegans would be better off eating live, plant-sourced foods and avoiding restaurants that serve foods with SOy products in them.
THERE IS A CURE FOR DIABETES by Gabriel Cousens MD
http://www.amazon.com/There-Cure-Diabetes-21-Day-Program/dp/1556436912
>0108 A healthy diet should consist of 80-percent-live and 20-percent-cooked food with a minimum of SOy products. SOy increases IGF-1 (Insulin Growth Factor-1) which could stimulate cancer. Milk also contains IGF-1 (but at a lower level than SOy) and for this reason, Canada does not allow rBGH milk from the U.S.
THERE IS A CURE FOR DIABETES by Gabriel Cousens MD
http://www.amazon.com/There-Cure-Diabetes-21-Day-Program/dp/1556436912
>0108 Infants on SOy formula had concentrations of isoflavones (estrogen-like substances) in their blood that were 13,000 to 22,000 times higher than the normal concentrations of natural estrogen.
THERE IS A CURE FOR DIABETES by Gabriel Cousens MD
http://www.amazon.com/There-Cure-Diabetes-21-Day-Program/dp/1556436912
>0108 SOy is low in essential minerals. In addition, SOy contains phytin, which removes the essential minerals from the body before they can be absorbed.
THERE IS A CURE FOR DIABETES by Gabriel Cousens MD
http://www.amazon.com/There-Cure-Diabetes-21-Day-Program/dp/1556436912
Now I only buy organic eggs and organic chicken, unless it is Marks & Spencer chicken from a supplier unique to M&S.
Problem solved. I find the eggs are okay whichever way I choose to cook them, yummy! ..." If we had had three comments within a week, how many more people were having chicken/egg related problems - and were they really right, that it was the SOy feed that was causing the problem?
Michelle Berriedale-Johnson investigates after the FM newsboard received three calls within about SOy residues within one week ... http://www.foodsmatter.com/Conditions/Allergy%20and%20Intolerance/Soya.in.chicken.html
>1107 SOy ingredients are in many of our foods. 66% of fat consumed comes from SOybean oil. SOy protein islolate from SOy meal is added to many of our foods. 98% of SOy grown is fed to livestock in the form of SOy meal. SOy is contaminated with organophophate pesticides. 87% of SOy is GMo (genetically engineered). One should use only certified organic SOy.
Dr. Alan Greene MD http://www.youtube.com/watch?v=IUHrj1Yv-1Y&feature=related
>1007 ‘SOy—Cause or Cure?
Things You Must Know about the Link Between Soy and Breast Cancer’
SOy protein contains dangerous levels of plant estrogens that have been shown to increase the risk for breast cancer.
http://www.wholesoystory.com/newsletters/Soy_BreastCancerAwarenessMonth.pdf
>1007 ‘SOy Foods Lower Sperm Count’
A study of 100 men showed that the men who consumed the highest levels of SOy foods had fewer sperm than men who did not consume any SOy.”
http://www.wholesoystory.com/newsletters/MaleFertility.pdf
>0907 ‘Oprah Diagnosed with Thyroid Disease—Are Soy Products to Blame?’
Oprah Winfrey announced that she "blew out" her thyroid raising the question of whether her diagnosis was the result of frequent consumption of SOy milk and other SOy products. Dr. Daniel states that for menopausal women, SOy is neither safe nor effective as a method of hormone replacement therapy. http://www.wholesoystory.com/newsletters/OprahDiagnosedWithThyroidDisease.pdf
>0907 Asians are not healthier people because they eat soy. Asians consume very little SOy. SOy was never considered a nutritional food. It was only consumed in times of famine when there was nothing else to eat. Asians eat fermented SOy which is easier to digest. The Chinese and Japanese eat approximately 2 teaspoons of fermented SOy a day. SOy is dangerous. It is one of the top most allergenic foods. SOy is cross reactive to wheat. SOy causes a range of inflammatory conditions. It is goitergenic. One glass of SOy milk affects the thyroid causing hypothyroidism. SOy increases the need for vitamin D. It is an inefficient form of B12. It has a trypsin inhibitor which blocks the absorption of protein and interferes with the function of the pancreas. SOy is estrogenic. Babies on SOy formula are taking the equivalent of 5 birth control pills a day. The estrogens in SOy cause early puberty in children and reproductive abnormalities. SOy is not recognized by the government as GRAS (Generally Recognized As Safe).
Dr. Sherrill Sellman http://www.youtube.com/watch?v=jWMs5EzXHVM&feature=related
>0507 ‘VEGAN PARENTS JAILED FOR BABY'S DEATH’
A six week old baby died of starvation after being fed SOy milk and apple juice.
http://www.wholesoystory.com/newsletters/SOYMILKdeaths2.pdf
>1206 SOy is one of the most processed foods out there. It is in many foods including SOy dogs, SOy burgers, SOy milk. SOy has phytic acid which leaches important nutrients from the body. Babies should never be given SOymilk. Babies should be breastfed. If you need an alternative, use raw milk, not pasteurized milk. SOy milk has estrogens. Male babies about 18 months old have a testosterone peak. The estrogens in soymilk interfere with the male hormones. Babies on SOy milk have estrogen levels 200 times the normal amount. Young girls with too much estrogen maturate early, reaching puberty at the age of 8 or 9. Many other products have estrogens, including detergents.
Dr. Ed Connaughton http://www.youtube.com/watch?v=s0ZLyFzmHyw&feature=related
> 1106 ‘FRENCH GOVERNMENT TO REQUIRE WARNING LABELS ON SOY FOODS’
“The French Food Agency (AFSSA) has announced tough new regulations that will require manufacturers to improve the safety of SOy infant formula and to put warning labels on packages of SOy foods and SOy milk.”
http://www.wholesoystory.com/newsletters/FrenchWARNING.pdf
>1106 ‘You Can’t Milk a SOy Cow’
SOy is one of the most genetically modified products out there. They've taken the SOybean and turned it into SOymilk, tofu, tofurky, SOy chips. Any kind of food you can think of now has SOy in it. SOy is considered the ultimate in health foods. The truth is that SOy is not good for women. It suppresses the thyroid function. SOy is not the reason that Asians are healthy. The truth is that in the 1930s, SOy intake was only 1.5% of their diet. 65% was pork. If you can go hunt down a SOycow and milk it, then great--use that for your soy source. If not, stick with whole healthy natural foods that you were meant to eat.
http://www.youtube.com/watch?v=7eGmIMYZiSQ&NR=1
>1006 believes that the increase in allergies and asthma in children may be caused by SOy and other GMo foods. The four genetically engineered crops are SOy, corn, cotton, and canola oil. Corn syrup and lecithin (SOy) is in almost everything. These foods have a lot stronger effect on children than on adults, because children are growing. Genetically engineered bovine growth hormone is given to cows and ends up in the milk, so many people are going to organic milk.
Deborah Koons Garcia http://www.youtube.com/watch?v=LhdRoyhoUj4
>0106 AMERICAN HEART ASSOCIATION REVERSES POSITION SOY HAS LITTLE EFFECT ON CHOLESTEROL
“The American Heart Association's decision will send shock waves through the SOy industry, which has profited mightily from marketing the myth that SOy prevents heart disease. Millions of Americans have been sold on the idea that is a heart-healthy food. In fact, scientists have long known that SOy does not reliably lower cholesterol and that it raises other heart disease risk factors, including homocysteine levels. Recently a study even showed that SOy worsens cardiomyopathy.“
http://www.wholesoystory.com/newsletters/AHAheartSOY.pdf
>0106 'there is a thyroid problem / SOy associated epidemic' ... Most SOy is GMo (genetically modified) unless it says “organic”. Non fermented SOy should be avoided (including Tofu). Fermented organic SOy (Tempeh, Miso, and Natto) is probably okay. SOy contains Trypsin inhibitors which interfere with protein digestion. SOy is loaded with goitergens which impair thyroid function. SOy contains phytic acid which impairs the ability to absorb minerals. SOy contains phytoestrogens which cause complications in young children and infants. SOy infant formulas should be banned and removed from the commercial market. They have found 20,000 times the amount of estrogen in the blood of babies on SOy infant formula compared to babies not on SOy. SOy formula contains toxic levels of manganese and aluminum. SOy protein isolate (aka textured vegetable protein) has high levels of MSG and aluminum.
Dr. Joseph Mercola
http://www.youtube.com/watch?v=RjZs0DGW1Jk&NR=1
>0106 Soy Alert!
“The dangers of SOy: High levels of phytic acid in soy reduce assimilation of minerals. Trypsin inhibitors in SOy interfere with protein digestion and may cause pancreatic disorders. SOy phytoestrogens disrupt endocrine function and have the potential to cause infertility and to promote breast cancer in adult women. SOy phytoestrogens are potent antithyroid agents that cause hypothyroidism and may cause thyroid cancer. In infants, consumption of SOy formula has been linked to autoimmune thyroid disease. Vitamin B12 analogs in SOy are not absorbed and actually increase the body’s requirement for B12. SOy foods increase the body’s requirement for vitamin D. Toxic synthetic vitamin D2 is added to soy milk. Fragile proteins are over-denatured during high temperature processing to make SOy protein isolate and textured vegetable protein. Processing of SOy protein results in the formation of toxic lysinoalanine and highly carcinogenic nitrosamines. Free glutamic acid or MSG, a potent neurotoxin, is formed during SOy food processing and additional amounts are added to many SOy foods to mask SOy’s unpleasant taste. SOy foods contain high levels of aluminum, which is toxic to the nervous system and the kidneys.”
www.westonaprice.org/brochures/SoyAlertTrifold.pdf
> 0805 The Promotion of Soy: “Early SOy food promotion in America aimed at two specific markets—vegetarians and the poor—Thus began the campaign to sell SOy products to the upscale consumer, not as a cheap poverty food, but as a miracle substance that would prevent heart disease and cancer, whisk away hot flashes, build strong bones and keep us forever young. SOy funds for research enlisted the voices of university professors who haplessly demonized the competition—meat, milk, cheese, butter and eggs.”
www.westonaprice.org/soy/promotion.html
>0705 ISRAELI HEALTH MINISTRY ISSUES SOY WARNING
Scientists, doctors and nutritionists who have warned that SOy is not a health food and poses special risks to infants and children
Daniel, K T. The Whole SOy Story http://www.wholesoystory.com/newsletters/IsraelSOYadvisory.pdf
CONSUMERS MISLED BY NATIONAL SOYFOODS MONTH SOY EXPERT WARNS OF MARKETING PLOY
The SOyfoods Association of North America touts SOy as the nutritious solution for children who are allergic to dairy products, peanuts and tree nuts. However, SOy is one of the top eight allergens, and SOy allergies are on the rise. The FDA now requires food manufacturers to list SOy ingredients clearly on food labels because of this danger.
Daniel, K T. The Whole SOy Story http://www.wholesoystory.com/newsletters/SoyFoodsMonth1.pdf
>0305 Scientists are not completely certain which components of SOy cause allergic reactions. They have found at least sixteen allergenic proteins, and some researchers pinpoint as many as thirty.
Daniel, K T. The Whole SOy Story. www.TheWholeSOyStory.cOM
>0305 “Children with severe allergy to peanut should avoid intake of SOy protein. To be on the safe side she further advised parents to make an effort to “avoid sensitization” by limiting both peanuts and SOybeans during the third trimester of pregnancy, during breast feeding, and by avoiding the use of SOy formula…A significant number of children with cow’s milk protein intolerance develop SOy protein intolerance when SOy milk is used in dietary management… a SOy protein component that cross reacts with caseins from cow’s milk. Cross reactions occur when foods are chemically related to each other…babies under three-months old who had reacted poorly to dairy formula also reacted to SOy formula.”
Daniel, K T. The Whole SOy Story http://www.wholesoystory.com/index.php?pageID=Media
>0305 “Allergic reactions occur not only when SOy is eaten but when SOybean flour or dust is inhaled…SOy dust is very asthmogenic and that asthma morbidity in a community can be influenced by exposures in the ambient atmosphere.”
http://www.wholesoystory.com/index.php?pageID=Excerpts
>0204 ‘Soy Alert: Soy: The Dark Side of America's Favorite’ --Sally Fallon and Mary Enig reveal the truth about soy in the average diet. SOy depresses thyroid function, increases the incidence of Alzheimer's and dementia, causes digestive problems and mineral deficiencies, and is dangerous for infants and children.
www.westonaprice.org/soy/darkside.html
>0104 Scientists have known since 1926 that some plants and herbs have a contraceptive effect. In the 1940s scientists discovered three plant estrogens in clover, a plant closely related to SOy. In female sheep, eating clover caused endometrial damage and cervical mucus changes which were associated with an inability to conceive.
http://www.thewholesoystory.com/BREEDING_DISCONTENT.pdf
>0104 ‘SOy goes by many aliases.’ such as “textured vegetable protein (TVP), “textured plant protein,” “hydrolyzed vegetable protein (HVP),” “vegetable protein concentrate,” “vegetable oil” or “MSG (monosodium glutamate).” Ingredient lists also include words such as “lecithin,” “vegetable oil,” “vegetable broth,” “boullion,” “natural flavor” or “mono-diglyceride” that do not necessarily come from SOy, but are likely to.
http://www.thewholesoystory.com/WHERE_THE_SOYS_ARE.pdf
>0104 SOy is hidden in thousands of everyday foods and items. This can be a challenge for people who are allergic to SOy as some people experience severe allergic reactions to even tiny amounts of soy. Those who are allergic to SOy must eliminate all SOy from their diets. http://www.thewholesoystory.com/WHERE_THE_SOYS_ARE.pdf
>0501 ‘SOy Alert! THE SOy CONTROVERSY’ describes how the US government is promoting SOy products as meat replacements for humans, while at the same time many prominent scientists are warning of the dangers of SOy. www.westonaprice.org/soy/soy_controversy.html
>0900 SOy is a powerful cancer promoter of the breast, prostate, lung, and colon. SOy is almost twice as powerful as milk protein in increasing IGF1 [insulin like growth factor 1] which contributes to cancer development.
J din Endocrinol Metah, March 2003, 88(3)1048—1054.
Yu, H. “Role of the insulin-like growth factor family in cancer development and progression.” J Natl Cancer Inst, Sept. 20, 2000, 92(18):1472-1489.
>0400 ‘Tragedy and Hype: The Third International SOy Symposium’ describes how SOy was marketed as a ‘miracle food’ to gain customer support. SOy is in almost everything from animal feed to processed foods and school lunch programs.
www.westonaprice.org/soy/tragedy.html
>0400 High midlife tofu consumption was associated with low brain weight. Brain atrophy was assessed in 574 men using MRI results and in 290 men using autopsy information. Shrinkage occurs naturally with age, but the men who had consumed more tofu showed an exaggeration of shrinkage.
White, L R, Petrovitch, H, Ross, G W, Masaki, K H, Hardman j, Nelson. J, Davis. D, and Markeshery, W. “Brain aging and midlife tofu consumption.” J Am Coll Nutr, April 2000, 19(2):242—255.
>0100 ‘Myths and Truths About SOy’-- The Weston A Price Foundation exposes the myths and explains the truth behind a number of soy fallacies:
Myth: SOy foods provide complete protein.
Truth: Like all legumes, SOy beans are deficient in sulfur-containing amino acids methionine and cystine. In addition, modern processing denatures fragile lysine.
Myth: Fermented SOy foods can provide vitamin B12 in vegetarian diets.
Truth: The compound that resembles vitamin B12 in SOy cannot be used by the human body; in fact, SOy foods cause the body to require more B12
Myth: SOy formula is safe for infants.
Truth: SOy foods contain trypsin inhibitors that inhibit protein digestion and affect pancreatic function. In test animals, diets high in trypsin inhibitors led to stunted growth and pancreatic disorders. SOy foods increase the body's requirement for vitamin D, needed for strong bones and normal growth. Phytic acid in SOy foods results in reduced bioavailabilty of iron and zinc which are required for the health and development of the brain and nervous system. SOy also lacks cholesterol, likewise essential for the development of the brain and nervous system. Megadoses of phytoestrogens in SOy formula have been implicated in the current trend toward increasingly premature sexual development in girls and delayed or retarded sexual development in boys.
Myth: Modern SOy foods protect against many types of cancer.
Truth: A British government report concluded that there is little evidence that SOy foods protect against breast cancer or any other forms of cancer. In fact, SOy foods may result in an increased risk of cancer.
Myth: SOy estrogens (isoflavones) are good for you.
Truth: SOy isoflavones are phyto-endocrine disrupters. At dietary levels, they can prevent ovulation and stimulate the growth of cancer cells. Eating as little as 30 grams (about 4 tablespoons) of SOy per day can result in hypothyroidism with symptoms of lethargy, constipation, weight gain and fatigue.
Myth: SOy foods are safe and beneficial for women to use in their postmenopausal years.
Truth: SOy foods can stimulate the growth of estrogen-dependent tumors and cause thyroid problems. Low thyroid function is associated with difficulties in menopause.
Myth: Phytoestrogens in SOy foods can enhance mental ability.
Truth: A recent study found that women with the highest levels of estrogen in their blood had the lowest levels of cognitive function; In Japanese Americans tofu consumption in mid-life is associated with the occurrence of Alzheimer's disease in later life.
Myth: SOy foods are good for your sex life.
Truth: Numerous animal studies show that soy foods cause infertility in animals. SOy consumption enhances hair growth in middle-aged men, indicating lowered testosterone levels. Japanese housewives feed tofu to their husbands frequently when they want to reduce his virility.
> 0100 Men who eat SOy isoflavones increase their chance of estrogen dominance leading to hair loss, swollen prostate glands and prostate cancer. A high SOy intake significantly reduces testicular function and lowers luteinizing hormone (LH) production, which is what signals the testicles to work.
Nagata, C, et al. “Inverse association of SOy product intake with serum androgen and estrogen in Japanese men.” Nutr Cancer, 2000, 36(1):14—18.
Zhong, Ying, et al. “Effects of dietary snpplement of SOy protein isolate and low fat diet on prostate cancer.” FASEB J, 2000, 14(4):a531.11.
>0100 Isoflavones decrease the good cholesterol (HDL), thus contributing to heart disease.
Asheon, E, and Ball, M. “Effects of SOy as tofu vs. meat on lipoprotein concentrations.” EurJ Cliii Nutr, Jan. 2000, 54(1):14—19,
Madani, 5, et al. “Dietary protein level and origin (casein and highly purihed SOybean protein) affect hepatic storage, plasma lipid transport, and antioxidative defense status in the rat.” Nutrition, May 2000, 16(S):368—375.
>1299 ‘The amount of phytoestrogens that are in a day’s worth of SOy infant formula equals 5 birth control pills,’ says Mike Fitzpatrick, a New Zealand toxicologist.
“SOy Infant Formula Could Be Harmful to Infants: Groups Want it Pulled.” Nutrition Week, Dec 10, 1999, 29(46):1—2; see also www.SOyonlineservice.co.nz.
>0199 ‘SOy Alert—SOy Infant Formula - Better than Breastmilk?’ describes the many dangers of SOy infant formula including allergies, nutritional deficiencies, retarded growth, brain and nervous system problems, sexual abnormalities, and cancer.
www.westonaprice.org/soy/infant.html
>0198 Female children fed the phytoestrogens in SOy formula reached puberty early, sometimes as young as age 6 to 8.
Irvine, C H G, Fitzpatrick, M G, and Alexander, S L. “Phytoestrogens in SOy based infant foods: Concentrations, daily intake and possible biological effects.” Proc Soc Exp Biol Med, 1998, 217:247—253.
>0797 The ‘daily exposure of infants to isoflavones in SOy infant-formulas is 6—11-- fold higher on a bodyweight basis than the dose that has hormonal effects in adults consuming SOy foods.’
Setchell, K D, Zimmer-Nechemias, L, Cai, J, and Heubi, J E. “Exposure of infants to phyto-oestrogens from SOy-based infant formula.” The Lancet, July 5, 1997, 350(9070):23—27.
> 0297 SOy contains isoflavones with built-in insecticides (genistein and daidzein). Isoflavones are estrogen-like substances that have the same effect in the body as estrogen. Eating SOy can contribute to irritability, mood swings, weight gain, uterine fibroids, fibrocystic breast disease, and cancer.
Casanova, M, et al. “Developmental effects of dietary phytoestrogens in Sprague: Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro.” Toxicol Sci, Oct. 1999, 51(2):236—244.
Santell, L, et al. “Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic / pituitary axis in rats.” J Nutr, Feb. 1997, 127(2):263—269.
Harrison, R M, et al. “Effect of genistein on steroid hormone production in the pregnant rhesus monkey.” Proc Soc Exp Biol Mcd, Oct. 1999, 222(1):78—84.
> 0796 SOy may also contribute to Alzheimer’s disease. In a major ongoing study involving 3,734 elderly Japanese American men, those who ate the most tofu during midlife had up to 2.4 times the risk of later developing Alzheimer’s disease. As part of the three-decade-long Honolulu-Asia Aging Study, twenty-seven foods and drinks were correlated with participants’ health. Men who consumed tofu at least twice weekly had more cognitive impairment than those who rarely or never ate the SOybean curd.
White, L R, Petrovich, H, Ross, G W and Masaki, K H. “Association of mid-life consumption of tofu with late life cognitive impairment and dementia: The Honolulu-Asia Aging Study.” Fifth International Conference on Alzheimer’s Disease, #487, 27 July 1996, Osaka, Japan.
White, L R, Petrovitch, H, Ross, G W, Masaki, K H, Hardman j, Nelson. J, Davis. D, and Markeshery, W. “Brain aging and midlife tofu consumption.” J Am Coll Nutr, April 2000, 19(2):242—255.
> 0196 Environmental and food estrogens, like those found in SOy, are responsible for the worldwide reduction in male fertility.
Rapp, D J. Is This Your Child’s World? New York: Bantam Books, 1996, p.SO1.
> 0995 ‘Soy Alert—The Ploy of Soy’ describes the history of soy in China. The Chinese consumed fermented foods such as tempeh, miso, natto, and shoyu. Contrary to popular opinion, unfermented SOy products were not consumed by the Chinese.
www.westonaprice.org/soy/ploy.html
> 0195 SOy isoflavones eaten during pregnancy may affect the sexual differentiation of the fetus toward feminization, cause malformations of the reproductive tract, or cause offspring to be born with both male and female sexual organs.
Levy, j R, Faber, F A, Ayyash, L, and Hughes, CL. “The effect of prenatal exposure to phytoestrogens genistein on sexual differentiation in rats.” Proc Soc Exp Biol Mcd, 1995, 208:60—66.
> 0994 The amount of phytoestrogens [estrogen-like substances] in two glasses of SOy milk per day is enough to change a woman’s menstrual patterns.
Cassidy, A, Bingham, 5, and Setchell, K D. “Biological effects of a diet of SOy protein rich in isoflavones on the menstrual cycle of premenopausal women.” AmJ Clin Nutr, Sept. 1994, 60(3):333—340.
> 0190 SOy contains Isoflavones [estrogen-like substances] which decrease thyroid hormone production. A hypothyroid condition can raise serum cholesterol, stunt a children’s growth, create fatigue, and lead to obesity.
Divi, R L, Chang, H C, and Doerge, D R. “Identification, characterization and mechanisms of anti-thyroid activity of isoflavones from SOybeans.” Biocheni PhartnacoL 1997, 54:1087—1096.
Fort, P, Moses, N, Fasano, M, Goldberg, T, and Lifshitz, E “Breast and SOy formula feedings in early infancy and the prevalence of autoimmune disease in children.” JAm Coil Nutr, 1990, 9:164—1 65.
Setchell, K D R, Zimmer-Nechemias, L, Cai, J, and 1-leubi, J E. “Exposure of infants to phytoestrogens from SOy based infant formula.” The Lancet, 1997, 350:23—27.
>0186 When it comes to allergies, SOy is one of the “sinister seven” and is widely known to cause immediate hypersensitivity reactions.
Berger, S. Dr. Berger’s Immune Power Diet. New York: New American Library,1986
Adrenalin Love ...
Sponsorship support ...
NicholasDynesGracey@ADRENALIN.org