"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
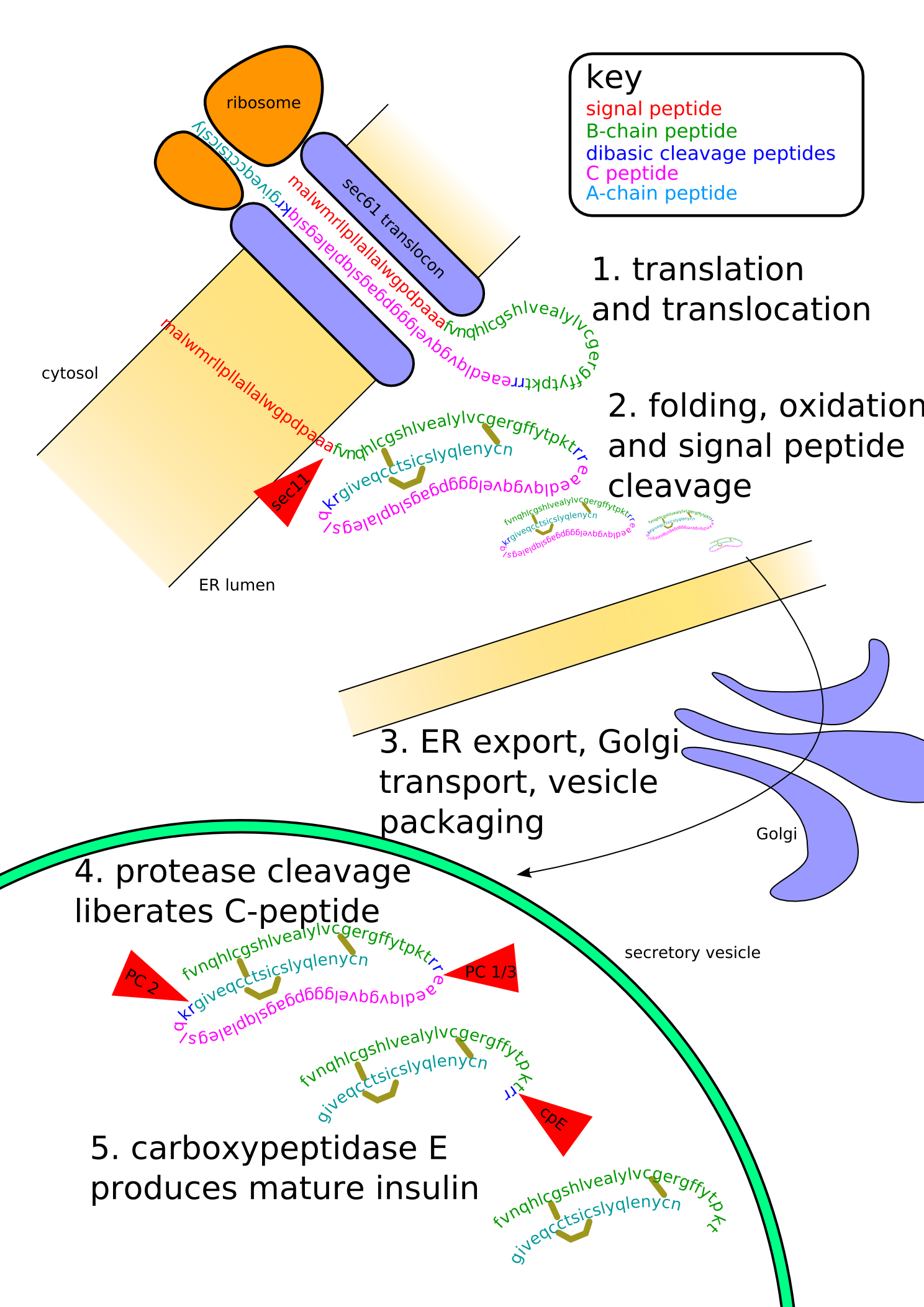
Proinsulin is the prohormone precursor to insulin made in the beta cell of the islets of Langerhans. It is synthesized in the endoplasmic reticulum, where it is folded and its disulfide bonds are oxidized. It is then transported to the Golgi apparatus where it is packaged into secretory vesicles, and where it is processed by a series of proteases to form mature insulin. Mature insulin has 39 fewer amino acids; 4 are removed altogether, and the remaining 35 form the C-peptide. The C-peptide is abstracted from the center of the proinsulin sequence; the two other ends (the B chain and A chain) remain connected by disulfide bonds.
When insulin was originally purified from bovine or porcine pancreata, all the proinsulin was not fully removed. When some people used these insulins, the proinsulin may have caused the body to react with a rash, to resist the insulin, or even to make dents or lumps in the skin at the place where the insulin was injected. In most cases however, the iatrogenic immune response comes from slight differences between species of insulin rather than from proinsulin itself. Since the late 1970's, when highly-purified pork insulin was introduced, and the level of insulin purity reached 99%, this ceased to be a significant clinical issue.
It should also be noted that in respect of their influence on insulin pharmacokinetics, moderate concentrations of certain insulin antibodies may, in fact, be of positive advantage to all diabetics without endogenous insulin secretion (e.g. people with type 1 diabetes) because insulin binding antibodies effectively increase the insulin's clearance rate and distribution space and therefore helps to prolong its pharmacological and biological half lives.
Insulin undergoes extensive posttranslational modification along the production pathway. Production and secretion are largely independent; prepared insulin is stored awaiting secretion. Both C-peptide and mature insulin are biologically active. Cell components and proteins in this image are not to scale...
2006 (C) Isaac Yonemoto
Proamylin Proislet Amyloid Polypeptide (proIAPP,Proamylin, Amyloid Polypeptide Precursor, Proislet Protein) Proislet amyloid polypeptide (proIAPP) is the protein precursor for Islet amyloid polypeptide (IAPP, amylin) (Higham et al., 2001).
ProIAPP is produced in the pancreatic beta cells (β-cells) as a 67 amino acid, 7404 Dalton pro-peptide and undergoes post-translational modifications to become the 37 amino acid IAPP hormone, also known as amylin. IAPP is cosecreted with insulin from the pancreatic β-cells in a ratio of approximately 100:1. Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
Once produced by the β-cells, it undergoes proteolysis. After this the transformation from the precursor protein proIAPP to the biologically active IAPP is complete.
Insulin and IAPP are regulated by similar factors since they share a common regulatory promoter sequence (Höppener, Ahrén, & Cornelius, 2000). One of the defining features of Type 2 diabetes is insulin resistance. This is a condition wherein the body is unable to utilize insulin effectively, resulting in increased insulin production; since proinsulin and proIAPP are cosecreted, this results in an increase in the production of proIAPP as well.
Although little is known about IAPP regulation, its connection to insulin indicates that regulatory mechanism that affect insulin also affect IAPP. Thus blood glucose levels play an important role in regulation of proIAPP synthesis.
ProIAPP has been linked to Type 2 diabetes and the loss of islet β-cells (Paulsson et al., 2005). Islet amyloid formation, initiated by the aggregation of proIAPP, may contribute to this progressive loss of islet β-cells. It is thought that proIAPP forms the first granules that allow for IAPP to aggregate and form amyloid which may lead to amyloid-induced apoptosis of β-cells.
IAPP is cosecreted with insulin. Insulin resistance in Type 2 diabetes produces a greater demand for insulin production which results in the secretion of proinsulin (Marzban, Rhodes, Haataja, Halban, & Verchere, 2006). ProIAPP is secreted simultaneously, however, the enzymes that convert these precursor molecules into insulin and IAPP, respectively, are not able to keep up with the high levels of secretion, ultimately leading to the accumulation of proIAPP.
In particular, the impaired processing of proIAPP that occurs at the N-terminal cleavage site is a key factor in the initiation of amyloid (Marzban et al., 2006). Post-translational modification of proIAPP occurs at both the carboxy terminus and the amino terminus, however, the processing of the amino terminus occurs later in the secretory pathway. This might be one reason why it is more susceptible to impaired processing under conditions where secretion is in high demand (Marzban et al., 2005). Thus, the conditions of Type 2 diabetes—high glucose concentrations and increased secretory demand for insulin and IAPP—could lead to the impaired N-terminal processing of proIAPP. The unprocessed proIAPP can then serve as the granule upon which IAPP can accumulate and form amyloid (Paulsson et al., 2006).
The amyloid formation might be a major mediator of apoptosis, or programmed cell death, in the islet β-cells (Paulsson et al., 2006). Initially, the proIAPP aggregates inside the cell. The proIAPP acts as a seed, collecting IAPP from neighboring cells and forming an intracellular amyloid. As the amyloid grows, it spreads outside of the cell. The extracellular amyloid begins to excrete IAPP to other cells, inducing similar amyloid formation in other β-cells. The overall effect is an apoptosis cascade initiated by the influx of ions into the β-cells.
In summary, impaired N-terminal processing of proIAPP is an important factor initiating amyloid formation and β-cell death. These amyloid deposits are pathological characteristics of the pancreas in Type 2 diabetes. However, it is still unclear as to whether amyloid formation is involved in or merely a consequence of type 2 diabetes (Marzban et al., 2006). Nevertheless it is clear that amyloid formation reduces working β-cells in patients with Type 2 diabetes. This suggests that repairing proIAPP processing may help to prevent β-cell death, thereby offering hope as a potential therapeutic approach for Type 2 diabetes.
Amylin, or Islet Amyloid Polypeptide (IAPP), is a 37-residue peptide hormone secreted by pancreatic β-cells at the same time as insulin (in a roughly 100:1 ratio).
Islet, or insulinoma, amyloid polypeptide (IAPP, or amylin) is commonly found in pancreatic islets of patients suffering diabetes mellitus type II, or harboring an insulinoma. While the assosciation of amylin with the development of type II diabetes has been known for some time, a direct causative role for amylin has been harder to establish. Recent results suggest that amylin, like the related beta-amyloid (Abeta) assosciated with Alzheimer's disease, can induce apoptotic cell-death in particular cultured cells, an effect that may be relevant to the development of type II diabetes.[1]
Amylin functions as part of the endocrine pancreas and contributes to glycemic control. Amylin's metabolic function is now somewhat well characterized as an inhibitor of the appearance of nutrient [especially glucose] in the plasma. It thus functions as a synergistic partner to insulin, with which it is cosecreted from pancreatic beta cells in response to meals. The overall effect to slow the rate of appearance (Ra) from the meal is mediated via a coordinate reduction of food intake, slowing of gastric emptying, inhibition of digestive secretion [gastric acid, pancreatic enzymes, and bile ejection].
Appearance of new glucose is slowed by inhibiting secretion of the gluconeogenic hormone glucagon. These actions, which are mostly mediated via a glucose-sensitive part of the brain stem, the area postrema, may be over-ridden during HYPOglycemia.
They collectively reduce the total insulin demand.[2][3]
Rodent amylin knockouts are known to fail to achieve the normal anorexia following food consumption. Because it is an amidated peptide, like many neuropeptides, it is believed to be responsible for the anorectic effect.
The human form of IAPP has the amino acid sequence KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY, with a disulfide bridge between cysteine residues 2 and 7. The peptide is secreted from the pancreas into the blood circulation and eventually excreted by the kidneys.
IAPP is capable of forming amyloid fibrils in vitro. Within the fibrillization reaction, the early prefibrillar structures are extremely toxic to insuloma cells cultures. Later amyloid fibril structures also seem to have some cytotoxic effect on cell cultures. Rats and mice have six substitutions (three of which are proline substitions at positions 25, 28 and 29) that are believed to prevent the formation of amyloid fibrils. Rat IAPP is unique since it is the only non fibril forming IAPP form.
IAPP was identified independently by two groups as the major component of diabetes-associated islet amyloid deposits in 1987.[4][5]
Synthetic amylin, or pramlintide (brand name Symlin), was recently approved for adult use in patients with both diabetes mellitus type 1 and diabetes mellitus type 2. Insulin and pramlintide, injected separately but both before a meal, work together to control the post-prandial glucose excursion.
There appears to be at least three distinct receptor complexes that bind with high affinity to amylin. All three complexes contain the calcitonin receptor at the core, plus one of three Receptor activity-modifying proteins, RAMP1, RAMP2, or RAMP3.