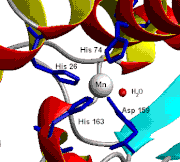"Every Day And In Every Way I Am Getting Better And Better"...
.
.
.
- Am J Hypertens. 2008 Jun;21(6):708-14. Epub 2008 Apr 10.

-
Hepatic effects of a fructose diet in the stroke-prone spontaneously HYPERtensive rat.
Center for Metabolic Disease, Ordway Research Institute, Albany, New York, USA.
BACKGROUND: Feeding stroke-prone spontaneously hypertensive rats (SHRSP) a diet rich in fructose results in a profound glucose intolerance not observed in the normotensive Wistar Kyoto (WKY) strain.
The aim of this study was to investigate the role of the liver in the underlying mechanisms in the SHRSP.
METHODS: SHRSP and WKY rats were fed either 60% fructose or regular chow for 2 weeks with blood pressure being measured using tail-cuff plethysmography and radiotelemetry.
Intraperitoneal glucose tolerance tests were performed and livers harvested for analysis of expression of inflammatory mediators and antioxidant proteins by western blotting and quantitative reverse transcriptase-PCR.
The serum triglyceride content and fatty acid profiles were also measured.
RESULTS: Feeding SHRSP and WKY on 60% fructose for 2 weeks resulted in glucose intolerance with no increases in levels of blood pressure.
Serum triglycerides were increased in both strains of fructose-fed rats with the highest levels being observed in the SHRSP.
The serum fatty acid profiles were changed with large increases in the amounts of oleic acid (18.1) and reductions in linoleic acid (18.2).
Levels of expression of c-jun N-terminal kinase/stress activated protein kinase (JNK/SAPK), and nuclear factor kappaB (NF-kappaB) were shown to be unchanged between the livers of the chow and fructose-fed groups.
In contrast, protein levels of the three isoforms of superoxide dismutase (SOD) were upregulated in liver of SHRSP fed on fructose while only manganese SOD (MnSOD) was upregulated in fructose-fed WKY rats.
CONCLUSIONS: These results demonstrate that the major contribution of the liver in the early pathogenesis of metabolic syndrome may be an increased secretion of triglyceride containing altered proportions of fatty acid pools.
Feeding rats a diet rich in fructose does not affect hepatic expression of inflammatory pathways and the increased hepatic SOD expression may constitute an early protective mechanism.
Feeding stroke-prone spontaneously hypertensive rats (SHRSP) a diet rich in fructose results in a profound glucose intolerance associated with increased hepatic SOD expression that is apparently profoundly protective.
Related Articles
-
- Skeletal muscle of stroke-prone spontaneously hypertensive rats exhibits reduced insulin-stimulated glucose transport and elevated levels of caveolin and flotillin. [Diabetes. 2001]
- The protective role of Kangen-karyu against fructose-induced metabolic syndrome in a rat model. [J Pharm Pharmacol. 2007]
- Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. [Circulation. 2004]
- Cardiopulmonary responses of Wistar Kyoto, spontaneously hypertensive, and stroke-prone spontaneously hypertensive rats to particulate matter (PM) exposure. [J Toxicol Environ Health A. 2007]
- Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. [J Hypertens. 2005]
- » See all Related Articles...
SuperOxide Dismutase
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
The enzyme SuperOxide Dismutase (SOD, EC 1.15.1.1), catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide. As such, it is an important antioxidant defense in nearly all cells exposed to oxygen. One of the exceedingly rare exceptions is Lactobacillus plantarum and related lactobacilli, which use a different mechanism.
Contents |
Reaction
The SOD-catalysed dismutation of superoxide may be written with the following half-reactions :
- M(n+1)+ − SOD + O2− → Mn+ − SOD + O2
- Mn+ − SOD + O2− + 2H+ → M(n+1)+ − SOD + H2O2.
where M = Cu (n=1) ; Mn (n=2) ; Fe (n=2) ; Ni (n=2).
In this reaction the oxidation state of the metal cation oscillates between n and n+1.
Types
General
SOD was discovered by Irwin Fridovich and Joe McCord, which prior were known as several metalloproteins with unknown function (for example, CuZnSOD was known as erythrocuprein). Several common forms of SOD exist: they are proteins cofactored with copper and zinc, or manganese, iron, or nickel.
Brewer (1967) identifed superoxide dismutase as an indophenol oxidase by protein analysis of starch gels using the phenazine-tetrazolium technique. Brewer detected this enzyme in several human tissues an indophenol oxidase A(IPO-A). After that Brewer observed an electrophoretic variant of IPO-A, which he called 'Morenci,' in 3 generations of a family with presumed male-to-male transmission. Using a RT-PCR analysis Brewer has identified 5 splice variants of SOD1. The variants were expressed in brain, a region involved in amyotrophic lateral sclerosis.
- The cytosols of virtually all eukaryotic cells contain an SOD enzyme with copper and zinc (Cu-Zn-SOD). (For example, Cu-Zn-SOD available commercially is normally purified from the bovine erythrocytes: PDB 1SXA, EC 1.15.1.1). The Cu-Zn enzyme is a homodimer of molecular weight 32,500. The two subunits are joined primarily by hydrophobic and electrostatic interactions. The ligands of copper and zinc are histidine side chains.
- Chicken liver (and nearly all other) mitochondria, and many bacteria (such as E. coli) contain a form with manganese (Mn-SOD). (For example, the Mn-SOD found in a human mitochondrion: PDB 1N0J, EC 1.15.1.1). The ligands of the manganese ions are 3 histidine side chains, an aspartate side chain and a water molecule or hydroxy ligand depending on the Mn oxidation state (respectively II and III).
- E. coli and many other bacteria also contain a form of the enzyme with iron (Fe-SOD); some bacteria contain Fe-SOD, others Mn-SOD, and some contain both. (For the E. coli Fe-SOD: PDB 1ISA, EC 1.15.1.1). Fe-SOD can be found in the plastids of plants. The active sites of Mn and Fe superoxide dismutases contain the same type of amino acid side chains.
- In higher plants, SOD isozymes have been localized in different cell compartments. Mn-SOD is present in mitochondria and peroxisomes. Fe-SOD has been found mainly in chloroplasts but has also been detected in peroxisomes, and CuZn-SOD has been localized in cytosol, chloroplasts, peroxisomes and
Human
In humans, three forms of superoxide dismutase are present. SOD1 is located in the cytoplasm, SOD2 in the mitochondria and SOD3 is extracellular. The first is a dimer (consists of two units), while the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, while SOD2 has manganese in its reactive centre. The genes are located on chromosomes 21, 6 and 4, respectively (21q22.1, 6q25.3 and 4p15.3-p15.1).
A microtiter plate assay for SOD is available.[3]
Biochemistry
Simply-stated, SOD outcompetes damaging reactions of superoxide, thus protecting the cell from superoxide toxicity. The reaction of superoxide with non-radicals is spin forbidden. In biological systems, this means its main reactions are with itself (dismutation) or with another biological radical such as nitric oxide (NO). The superoxide anion radical (O2-) spontaneously dismutes to O2 and hydrogen peroxide (H2O2) quite rapidly (~105 M-1 s-1 at pH 7). SOD is biologically necessary because superoxide reacts even faster with certain targets such as NO radical, which makes peroxynitrite. Similarly, the dismutation rate is second order with respect to initial superoxide concentration. Thus, the half-life of superoxide, although very short at high concentrations (e.g. 0.05 seconds at 0.1mM) is actually quite long at low concentrations (e.g. 14 hours at 0.1 nM). In contrast, the reaction of superoxide with SOD is first order with respect to superoxide concentration. Moreover, superoxide dismutase has the fastest turnover number (reaction rate with its substrate) of any known enzyme (~109 M-1 s-1)[citation needed], this reaction being only limited by the frequency of collision between itself and superoxide. That is, the reaction rate is "diffusion limited".
Physiology
Superoxide is one of the main reactive oxygen species in the cell and as such, SOD serves a key antioxidant role. The physiological importance of SODs is illustrated by the severe pathologies evident in mice genetically engineered to lack these enzymes. Mice lacking SOD2 die several days after birth, amidst massive oxidative stress.[4] Mice lacking SOD1 develop a wide range of pathologies, including hepatocellular carcinoma,[5] an acceleration of age-related muscle mass loss,[6] an earlier incidence of cataracts and a reduced lifespan. Mice lacking SOD3 do not show any obvious defects and exhibit a normal lifespan
Role in disease
Mutations in the first SOD enzyme (SOD1) have been linked to familial amyotrophic lateral sclerosis (ALS, a form of motor neuron disease). The other two types have not been linked to any human diseases, however, in mice inactivation of SOD2 causes perinatal lethality[4] and inactivation of SOD1 causes hepatocellular carcinoma.[5] Mutations in SOD1 can cause familial ALS, by a mechanism that is presently not understood, but not due to loss of enzymatic activity or a decrease in the conformational stability of the SOD1 protein. Overexpression of SOD1 has been linked to Down's syndrome.[7] The veterinary antiinflammatory drug "Orgotein" is purified bovine liver superoxide dismutase.
Delivery systems
Superoxide dismutase is effective as a nutritional supplement when bound to the polymeric films of wheat matrix gliadin (a delivery method also known as glisodin). Gliadin is an ideal carrier because it protects SOD from stomach acid and enzymes found in the digestive system which break down its molecular structure. This has been established in a variety of animal studies and human clinical trials, in which SOD's generally high antioxidant capacity is kept intact under a variety of conditions.
Cosmetic uses
SOD is used in cosmetic products to reduce free radical damage to skin, for example to reduce fibrosis following radiation for breast cancer. Studies of this kind must be regarded as tentative however, as there were not adequate controls in the study including a lack of randomization, double-blinding or placebo.[8] Superoxide dismutase is known to reverse fibrosis, perhaps through reversion of myofibroblasts back to fibroblasts.[9]
References
- ^ Corpas FJ, Barroso JB, del Río LA. (2001). "Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells.". Trends Plant Sci. 6 (4): 145–50..
- ^ Corpas FJ et al. (2006). "The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves.". Plant Cell Physio. 47 (7): 984–94.
- ^ A.V. Peskin, C.C. Winterbourn (2000). "A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1)". Clinica Chimica Acta 293: 157–166. doi:.
- ^ a b
 Li, et al., Y. (1995). "Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase.". Nat. Genet. 11: 376–381. doi:.
Li, et al., Y. (1995). "Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase.". Nat. Genet. 11: 376–381. doi:. - ^ a b
 Elchuri, et al., S. (2005). "CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life.". Oncogene 24: 367–380. doi:.
Elchuri, et al., S. (2005). "CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life.". Oncogene 24: 367–380. doi:. - ^
 Muller, et al., F. L. (2006). "Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy.". Free Radic. Biol. Med 40: 1993–2004. doi:.
Muller, et al., F. L. (2006). "Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy.". Free Radic. Biol. Med 40: 1993–2004. doi:. - ^
 Groner, Y. et al. (1994). "Cell damage by excess CuZnSOD and Down's syndrome.". Biomed Pharmacother. 48: 231–40. doi:. PMID 7999984.
Groner, Y. et al. (1994). "Cell damage by excess CuZnSOD and Down's syndrome.". Biomed Pharmacother. 48: 231–40. doi:. PMID 7999984. - ^
 Campana, F. (2004). "Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis" (available free). J. Cell. Mol. Med. 8 (1): 109–116. doi:. PMID 15090266.
Campana, F. (2004). "Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis" (available free). J. Cell. Mol. Med. 8 (1): 109–116. doi:. PMID 15090266. - ^ Vozenin-Brotons MC; Sivan V, Gault N, Renard C, Geffrotin C, Delanian S, Lefaix JL, Martin M (January 1, 2001). "Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts". Free Radic Biol Med 30 (1): 30–42. Elsevier. doi:. PMID 11134893.
See also
External links
- Online 'Mendelian Inheritance in Man' (OMIM) 105400 (ALS)
- The ALS Online Database
- A short but substantive overview of SOD and its literature.
- Damage-Based Theories of Aging Includes a discussion of the roles of SOD1 and SOD2 in aging.
- Physicians' Comm. For Responsible Med.
- SOD and Oxidative Stress Pathway Image
- Historical information on SOD research"The evolution of Free Radical Biology & Medicine: A 20-year history" and "Free Radical Biology & Medicine The last 20 years: The most highly cited papers"
- JM McCord discusses the discovery of SOD
| ||